Potentials and Constraints for the application of CFD to dry powder inhalers
Ansprechpartner:
Prof. Dr.-Ing. Habil Martin Sommerfeld
Abstract
Based on numerical simulations by the Euler/Lagrange approach flow field and particle transport through inhaler devices are studied. Based on that, the mechanisms of drug detachment from carrier particles are analysed and possible reasons for the bad practical performance are elucidated. Numerical results are presented for carrier particle and drug powder motion and deposition for different flow rates. The assets and drawbacks of the two mostly used particle tracking methods are highlighted and the needs for further model developments are presented.
Introduction
Dry powder inhalers (DPI) are increasingly used to administer drugs in the form of fine powders through the airways. These drugs are also called API (active pharmaceutical ingredient). Since such fine powders need to be smaller than about 5 µm they are very cohesive and difficult to handle. Therefore, two kinds of formulations are in use. Most common is the blending of larger carrier particles with the fine drugs (called cluster), however also agglomerated drug powder may be delivered to the inhaler. The main task of the inhaler is the detachment of the drug from the carrier or the destruction (aerosolisation) of the agglomerates. This is realized by flow stresses produced by the breathing-induced air flow or by frequent wall impact. Unfortunately, the efficiency of dry powder inhalers is very low and only 20 to 40 % of the fine particle dose is delivered to the lung.
Because of this deficiency in DPI application, computational fluid dynamics (CFD) is applied for more than one decade for analysing DPI related specific phenomena. Some of this work has been reviewed by Wong et al. (2012), Ruzycki et al. (2013) and Cui and Sommerfeld (2018). A detailed review was recently provided by Sommerfeld et al. (2019). The final objective for a numerical calculation of inhaler devices should the prediction of the emitted fine particle fraction from the inhaler and allow thereby for an optimisation of inhaler geometry and operational conditions. This is however not a straight forward task when it comes to the modelling of all involved transport processes and particle-scale phenomena occurring in such inhalers appropriately.
The complex and highly turbulent, mostly swirling flow field within inhalers can be nowadays effectively calculated using LES (Large eddy simulations) which only require rather simple models for the unresolved turbulence. Most of the simulations are however still done on the basis of RANS (Reynold-averaged Navier-Stokes) equations in connection with an appropriate turbulence closure, wherefore the k-w-SST model was found to be superior (see Sommerfeld et al. 2019). Until now quite a number of studies were related to Lagrangian point-particle calculations of the motion of different kinds of particles through inhaler devices, such as: fine drug particle motion behaviour (Coates et al. 2004); fine particle tracking and analysis of deposition in inhaler devices (Milenkovic et al. 2013) and analysis of coarse carrier particle motion in a Lagrangian way (see Donovan et al. 2012, Sommerfeld and Schmalfuß 2016). Since in technical equipment mostly a large number of particles have to be tracked their size and shape cannot be resolved numerically as it becomes very expensive. Therefore, the particles have to be treated as point-masses, requiring appropriate correlations and closures for the fluid dynamic forces and models for other particle-scale transport processes.
In the following section the different Lagrangian tracking methods will be analysed with regard to their applicability in DPI simulations.
Lagrangian particle models
The transport of particles (i.e. clusters, agglomerated drugs or drug particles) in DPIs is mostly calculated by Lagrangian point-particle methods using either the DPM (discrete particle method) or the DEM (discrete element method). The features of both methods are briefly summarised in the following (Sommerfeld et al. 2019):
- Regarding the discrete element method (DEM), particles are moved through the flow by considering fluid and external forces (e.g. gravity) as well as contact forces between particles (or between particles and walls) through a soft-sphere interaction model. Consequently, this approach allows for multiple particle contacts and seems to be ideal for calculating cluster and agglomerate transport. However, all particles in an agglomerate or cluster are tracked individually as point-masses and therefore all relevant fluid dynamic forces have to be corrected for swarm effects (high local particle volume fraction). This procedure is well established in DEM for the drag force (Zhu et al. 2007), but the question is how to treat transverse lift forces in shear flow or due to cluster rotation. Moreover, the question is which are the forces acting on a API situated on a carrier particle.
- In the discrete particle method (DPM) point-particles are tracked through the flow field bases on fluid dynamic and external (e.g. gravity) forces. Collisions between particles (or particles and walls) are treated as instantaneous binary collisions, also called hard-sphere collisions. Therefore, the calculation of cluster or agglomerate motion requires additional models, as for example the agglomerate structure model introduced by Sommerfeld and Stübing (2017). This method allows applying the fluid dynamic forces on the entire cluster and hence can account realistically for transverse lift forces due to rotation and shear. Particle-scale processes, such as flow detachment of drug particles or wall collisions of clusters, have to be described through additional models.
Hence, the point-particle DEM is most suitable for analysing particle scale processes without flow such as the collision between clusters or cluster-wall collisions (Ariane et al. 2018). Thereby models could be developed for being used in a DPM Lagrangian tracking. Such a multi-scale approach was applied by Tong et al. (2015) and van Wachem et al. (2017) for predicting the fine particle release from different types of inhalers. Both studies also included the possibility of drug powder deposition on the walls of the inhalers. The models (i.e. purely empirical fitting correlations) for flow-induced and wall-collision detachment were derived by conducting DEM simulations for a single carrier occupied with hundreds of fine drug particles.
Calculations on drug detachment from carrier
The numerical studies presented here focus on carrier-based formulations where a larger spherical particle is blended with thousands of fine drug particles. For analysing carrier particle motion through an inhaler RANS (for the present results the k-w-SST model) simulations were conducted using the DPM method. The developed advanced Lagrangian tracking includes all relevant forces acting on the particles, namely, drag, transverse lift due to shear and particle rotation, added mass and of course gravity/buoyancy. From tracking 1000 carrier particles through a Cyclohaler the PDFs (probability density functions) of the fluid stresses acting on the carrier particles were evaluated (Sommerfeld and Schmalfuß 2016). Additionally, these simulations were used to derive carrier-wall collision statistics including the number of wall collisions, impact locations and impact velocities as well as angles. At a flow rate of 100 l/min a 110 mm carrier particle experiences 100 wall collisions in a Cyclohaler, suggesting that wall collisions are very effective for drug detachment. Moreover, these studies showed the huge importance of transverse lift forces for the motion of carriers through the narrow passages of inhalers (Sommerfeld and Schmalfuß 2016).
The recorded fluid stresses (relative velocity, turbulence and shear stresses) acting on the carrier were used to perform fully resolved Lattice-Boltzmann simulations (e.g. 110 mm carrier with 882 drug particles of 5 mm). The results showed that flow detachment is of minor importance and may only happen through sliding or rolling (see Cui et al. 2014; Cui and Sommerfeld 2015). Turbulence in addition may remarkably enhance drug particle lift-off (Cui and Sommerfeld 2016).
In order to allow the determination of drug particle detachment from a carrier upon wall collision a novel model was derived in the frame of DPM (see Cui and Sommerfeld 2019) Euler/Lagrange simulations of the Cyclohaler. Applying this model revealed that almost 100 % of the drug particles were detached through the numerous wall impacts (Sommerfeld et al. 2019).
This is of course in contradiction to the experimental observation that only 20 – 40 % of the loaded drug particles are able to leave the inhaler. The answer to this discrepancy is the effect of drug particle deposition on the walls of the inhaler device. Since the drug particles are very small, wall adhesion is very strong and therefore re-dispersion is very difficult. Wall deposition may occur during transport of fine powders through the inhaler (Milenkovic et al. 2013), but also on the occasion of carrier-wall impacts as demonstrated by Ariane et al. (2018) through DEM simulations.
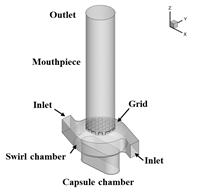
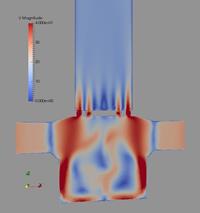
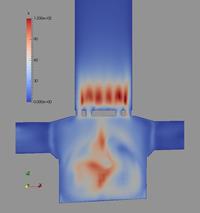
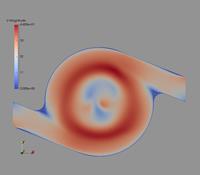
In the following, some of the results are presented showing the potential of the computations considering a Cyclohaler as an example. The geometry of the inhaler is shown in Figure 1 a) consisting of a capsule and a swirl chamber, a grid as a kind of flow straightener and the mouth piece. This real geometry was discretised by 1.44 million computational cells yielding the flow field illustrated in Figure 1 b) for a stationary flow rate of 60 l/min. It should be emphasised that the flow velocity magnitude reaches values above 50 m/s in the swirl chamber.
The carrier particles (here 1024 were considered) were released from the capsule chamber initially distributed homogeneously with constant separation. As soon as the particles are exposed to the flow field they are accelerated and begin to move out of the capsule and swirl chamber. The carrier particle (here 100 mm) distribution with increasing time is illustrated in Figure 2 where the colour of the particles indicated their Reynolds number (i.e. blue low Re = 10 and red high Re =200).
 |
 |
 |
 |
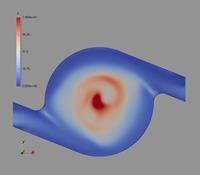 |
Figure 1: Flow field results for the Cyclohaler; a) configuration with inlets, capsule and swirl chambers, grid and mouth piece; b) Velocity magnitude (upper graphs) and turbulent kinetic energy (lower graphs) in a vertical cut as well as the cross-section of the swirl chamber (flow rate 60 l/min).
From such simulations many data may be extracted, such as the flow stresses acting on the carrier particles (Sommerfeld and Schmalfuß 2016) and the wall collision statistics experienced by a single carrier particle on the way through the inhaler as illustrated in Figure 3. The general trend is that the number of wall collisions decreases of course with reducing flow rate and mostly also with growing particle size (Figure 3 a). For a stationary flow rate of 60 l/min the wall collision number for one carrier on the entire way through the inhaler is around 200 and slightly deceased with size. The number of wall collisions in the different regions of the inhaler is shown in Figure 3 b). The lowest numbers are found in the mouth piece since the swirls was reduced through the grid. The most frequent wall collisions are of course found in the swirl and capsule chambers, as the carriers move within a strong rotating flow and are centrifuged outwards to the walls. It should be emphasised, that carrier-wall collisions are very effective in drug particle detachment from the carrier (Cui and Sommerfeld 2019) and as a consequence of the frequent wall collisions most fine drugs (or also called API) should have been detached. This however cannot explain the bad performance of such inhalers; i.e. only 20 – 40 % of the loaded drug particles are able to leave the inhaler. Consequently there must be another effect for the API not being released from the inhaler.
| 1ms | 3ms | 4ms | 5ms |
 |
 |
 |
 |
| 6ms | 7ms | 9ms | 10ms |
 |
 |
 |
 |
| Figure 2: Temporal evolution of the carrier particle (here 100 mm) distribution at a flow rate of 60 l/min (the actual time from the beginning of the computation is provided above the graphs). | |||
![2020-11-23 09_35_50-Report-CFD-Inhaler-2020-Sommerfeld.docx [Kompatibilitätsmodus] - Word 2020-11-23 09_35_50-Report-CFD-Inhaler-2020-Sommerfeld.docx [Kompatibilitätsmodus] - Word](/mps_media/Bilder/Forschungsbereich/Projekte/2020_11_23+09_35_50_Report_CFD_Inhaler_2020_Sommerfeld_docx+%5BKompatibilit%C3%A4tsmodus%5D+_+Word-height-252-width-315-p-502.png) |
![2020-11-23 09_36_06-Report-CFD-Inhaler-2020-Sommerfeld.docx [Kompatibilitätsmodus] - Word 2020-11-23 09_36_06-Report-CFD-Inhaler-2020-Sommerfeld.docx [Kompatibilitätsmodus] - Word](/mps_media/Bilder/Forschungsbereich/Projekte/2020_11_23+09_36_06_Report_CFD_Inhaler_2020_Sommerfeld_docx+%5BKompatibilit%C3%A4tsmodus%5D+_+Word-height-254-width-302-p-504.png) |
| Figure 3: Dependence of single carrier particle wall collision number on particle size and flow rate for a Cyclohaler (a); wall collision number within the different regions of a Cyclohaler in dependence of carrier particle diameter. | |
So far only the motion of carrier particles was studied. As mentioned above, deposition of API could be a reason for the bad performance of inhalers. Therefore, also motion and deposition of fine particles was analysed. In this case 2500 fine particles of different size were released from the capsule chamber. If the small particles hit a wall it was now also inspected whether deposition takes place or not. This check was based on a critical impact velocity determined by two approaches, an energy approach (Hiller 1981) and a JKR-based ansatz (Brach and Dunn 1992). Both methods gave actually about the same trends in the deposition efficiencies, but Hiller (1981) energy-based model provided lower values. The biggest problem with deposition modelling is the need of additional parameters and material properties which are mostly not available. The deposition rate increases with particle size and expectedly also with the flow rate and is for small particles below about 1 mm around 20 – 40 % implying that 60 – 80 % of such fine drug particles should leave the inhaler as illustrated in Figure 4. When considering all particles until about 5 mm (i.e. those particles which may deposit in the lung) the fine particle release rate is obviously much lower (i.e. with 5 mm APIs the deposition is about 80 % and hence a release rate of 20 % is the result). The restitution ratio e indicates the available rebound energy. For the case of e = 0.7 the energy dissipated in a wall collision is actually 30 %. Consequently if the dissipated energy increases (i.e. the value of e reduces) then deposition rate also is growing (Figure 4 a). Expectedly, different deposition models and different restitution ratios or model parameters yield different deposition rates. Therefore, experimental information on the material properties (i.e. inhaler and API) and wall collision behaviour is urgently needed. As to be expected with growing flow rate and resulting higher API impact velocities deposition rate also increase slightly (Figure 4 b).
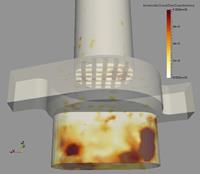
Moreover, the numerical computations allow also analysing the spatial location of deposition within the inhaler (Figure 5). As mentioned above, first deposition increases with particle size. Within the mouth piece wall collisions are rare and consequently also deposition is very low. Most of the deposition is found in the capsule and swirl chamber with some variations with respect to particle size. For the smallest particles a large amount is depositing on the grid to the mouth piece (Figure 5). For the 2 mm particles, deposition is highest in the capsule chamber and for the even larger 5 mm particles the highest deposition seems to be in the swirl chamber. A very small amount is deposited on the grid. Such information now may be used for optimising inhaler geometry in order to modify flow structure and finally deposition rates.
![2020-11-23 09_44_22-Report-CFD-Inhaler-2020-Sommerfeld.docx [Kompatibilitätsmodus] - Word 2020-11-23 09_44_22-Report-CFD-Inhaler-2020-Sommerfeld.docx [Kompatibilitätsmodus] - Word](/mps_media/Bilder/Forschungsbereich/Projekte/2020_11_23+09_44_22_Report_CFD_Inhaler_2020_Sommerfeld_docx+%5BKompatibilit%C3%A4tsmodus%5D+_+Word-height-233-width-339-p-506.png) |
![2020-11-23 09_44_46-Report-CFD-Inhaler-2020-Sommerfeld.docx [Kompatibilitätsmodus] - Word 2020-11-23 09_44_46-Report-CFD-Inhaler-2020-Sommerfeld.docx [Kompatibilitätsmodus] - Word](/mps_media/Bilder/Forschungsbereich/Projekte/2020_11_23+09_44_46_Report_CFD_Inhaler_2020_Sommerfeld_docx+%5BKompatibilit%C3%A4tsmodus%5D+_+Word-height-237-width-340-p-508.png) |
| Figure 4: Fine particle total deposition rate within the Cyclohaler as a function of particle size; a) for different deposition models and model parameters; b) for different flow rates. | |
| 1µm | 2µm | 5µm |
 |
 |
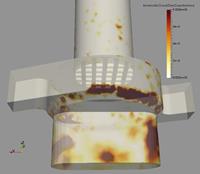 |
| Figure 5: Fine particle deposition rate within the lower part of the Cyclohaler for different particle diameters (flow rate 90 l/min). | ||
Conclusions
The DPM is most suitable for calculating particle motion through an inhaler since both clusters and agglomerates are treated as an entity and the relevant fluid dynamic forces can be accounted for correctly. However, all particle-scale processes such as drug detachment from a carrier, agglomerate breakage or wall impacts need to be described through realistic models. Essential for predicting the emitted fine particle fraction is also the deposition of drugs on the inhaler walls through flow transport as well as wall impacts.
Separate numerical predictions of carrier particle behaviour as well as fine particle transport and deposition allowed already drawing helpful conclusions regarding the possibilities optimising inhaler geometry. The next step will be the computation of carrier particle motion including the allocation with drug particles and therefore incorporating all models for API detachment and a possible wall deposition.
Acknowledgments
Financial support: DFG in the frame of the priority research program SPP 1486 (PiKo) under contract No. SO 204/38 1-3. Cooperation: COST Action MP 1404 “SimInhale” (European Cooperation in Science and Technology; www.cost.eu).
References
Ariane, M., Sommerfeld, M., Alexiadis, A.: Wall collision and drug-carrier detachment in dry powder inhalers: Using DEM to devise a sub-scale model for CFD calculations. Powder Technology, Vol. 334, 65 – 75 (2018)
Brach, R.M. and Dunn, P.F.: A mathematical model of the impact and adhesion of microspheres. Aerosol Science and Technology, Vol. 16, 51 – 64 (1992)
Coates, M.S., Fletcher, D.F., Chan, H.-K., Raper, J.A.: Effect of design on the performance of a dry powder inhaler using computational fluid dynamics. Part 1: Grid structure and mouthpiece length. Journal of Pharmaceutical Sciences, Vol. 93, 2863 – 2876 (2004)
Cui, Y., Schmalfuß, S., Zellnitz, S., Sommerfeld, M., Urbanetz, N.: Towards the optimisation and adaptation of dry powder inhalers. Int. Journal of Pharmaceutics, Vol. 470, 120 – 132 (2014)
Cui, Y., Sommerfeld, M.: Forces on micron-sized particles randomly distributed on the surface of larger particles and possibility of detachment. Int. Journal Multiphase Flow, Vol. 72, 39 – 52 (2015)
Cui, Y., Sommerfeld, M.: Application of Lattice-Boltzmann Method for Analysing Detachment of Micron-Sized Particles from Carrier Particles in Turbulent Flows. Flow Turbulence and Combustion, Vol. 100, 271 – 297 (2018)
Cui, Y., Sommerfeld, M.: The modelling of carrier-wall collision with drug particle detachment for dry powder inhaler applications. Powder Technology, Vol. 344, 741 – 755 (2019)
Donovan, M.J., Kim, S.H., Raman, V. & Smyth, H.D.: Dry powder inhaler device influence on carrier particle performance. Journal of Pharmaceutical Sciences, Vol. 101, 1097 – 1107 (2012)
Hiller, R.B.: Der Einfluss von Partikelstoß und Partikelhaftung auf die Abscheidung in Faserfiltern, Dissertation, Universität Karlsruhe, VDI-Verlag GmbH Düsseldorf, Düsseldorf (1981)
Milenkovic, J., Alexopoulos, A.H., Kiparissides, C.: Flow and particle deposition in the Turbohaler: A CFD simulation. International Journal of Pharmaceutics Vol. 448, 205 – 213 (2013)
Ruzycki, C.A., Javaheri, E., Finlay, W.H.: The use of computational fluid dynamics in inhaler design. Expert Opinion Drug Delivery, Vol. 10, 307 – 323 (2013)
Sommerfeld, M., Schmalfuß, S.: Numerical analysis of carrier particle motion in dry powder inhaler. ASME Journal of Fluid Engineering, Vol. 138, Paper 041308 (2016).
Sommerfeld, M., Stübing, S.: A novel Lagrangian agglomerate structure model. Powder Technology, Vol. 319, 34 – 52 (2017)
Sommerfeld, M., Cui, Y. and Schmalfuß, S.: Potentials and Constraints for the Application of CFD Combined with Lagrangian Particle Tracking to Dry Powder Inhalers. European Journal of Pharmaceutical Sciences, Vol. 128, 299 – 324 (2019)
Tong, Z., Kamiya, H., Yu, A., Chan, H.-K., Yang, R.: Multi-scale modelling of powder dispersion in a carrier-based inhalation system. Pharm. Res. Vol. 32, 2086 – 2096 (2015)
van Wachem, B., Thalberg, K., Remmelgas, J., Niklasson-Björn, I.: Simulation of dry powder inhalers: Combining micro-scale, mesco-scale and macro-scale modeling. AIChE J., Vol. 63, 501- 516 (2017)
Wong, W., Fletcher, D.F., Traini, D., Chan, H.-K., Yong, P.M.: The use of computational approaches in inhaler development. Advanced Drug Delivery Reviews, Vol. 64, 312 – 322 (2012)
Zhu, H.P., Zhou, Z.Y., Yang, R.Y., Yu, A.B.: Discrete particle simulation of particulate systems: Theoretical developments. Chemical Engineering Science, Vol. 62, 3378 – 3396 (2007)





